Galvanic Corrosion – What it is and how to prevent it?
Galvanic corrosion (also called bimetallic corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte. This occurs in batteries for example where the cathode stays whole and the anode corrodes as the battery is working. Contrary to some believes – Galvanic corrosion does not only occur in water. Galvanic cells can form in any electrolyte, including moist air or soil, and in chemical environments. As an example, Over 200 years ago, the British naval frigate Alarm lost its copper sheeting due to the rapid corrosion of the iron nails used to fasten copper to the hull. The electrolyte in this case was salt water creating a galvanic cell.

In the case of the Alarm, the iron acted as an anode and was corroded at the expense of the copper which acted as the cathode. Just two years after attaching the copper sheets, the iron nails that were used to hold the copper to the ship’s underside were already severely corroded, causing the copper sheets to fall off.
Metals and metal alloys all possess different electrode potentials. Electrode potentials are a relative measure of a metal’s tendency to become active in a given electrolyte. When in the same environment, the more active a metal is likely it is to form positively charged electrode (anode) and the less active metal is more likely it is to form a cathode (negatively charged electrode).
The electrolyte acts as a conduit for ion migration, moving metal ions from the anode to the cathode. The anode metal, as a result, corrodes more quickly than it otherwise would, while the cathode metal corrodes more slowly and, in some cases, may not corrode at all.
The Nex Flow Difference Allowing Products to Last Longer than Competitors
While products such as air knives, air nozzles, air amplifiers, vortex tubes, etc. are not necessarily immersed in any electrolyte, if the environment is humid, or if the equipment is subject to wash down procedures, it is very possible that this type of corrosion can occur. One example is mixing stainless steel and aluminum. There was one example where a competitive cabinet enclosure cooler was observed with a big hole on its side after some years of use. The stainless steel vortex tube inside combined with the aluminum housing, and the factory environment, over time caused the aluminum to act as an anode and started to corrode.
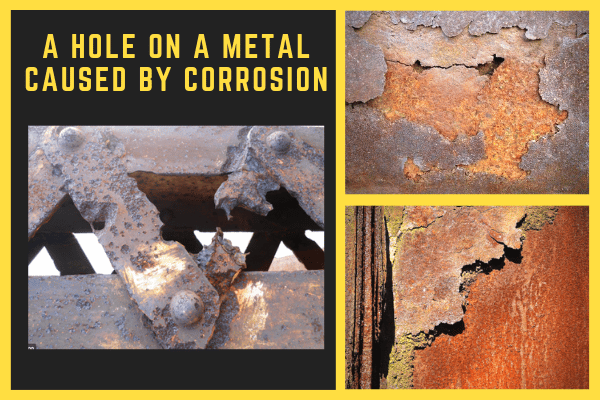
Nex Flow® takes certain steps and actions to prevent this from happening in their products allowing the products to last longer. The first is to protect aluminum that is used, especially if combining it with steel or stainless steel. Our aluminum air knives for example – are anodized and as such have a protective coating to prevent an “electrical circuit” with the stainless steel shims used inside and the stainless steel screws used to hold the air knife together. In addition, the aluminum would tend to act as an anode anyway in an electrolytic environment and being so large compared to the stainless steel shim corrosion would be minimized. Regardless of whether galvanic corrosion would occur, the anodization also protects the air knife from any environment which bare aluminum is unprotected. Similarly, Nex Flow anodizes all their aluminum parts – air knives, air jets, nozzles, air wipes and air operated conveyors such as the Ring vacs. Air amplifiers and flat jet nozzles which are aluminum zinc-cast are powder coated for longer life and also look better.
When it comes to vortex tube technology, such as cabinet enclosure coolers (panel coolers) and tool coolers, no aluminum is used. It is stainless steel with some brass internal parts. This works to ensure that you will not find any holes in Nex Flow Panel Coolers caused by galvanic corrosion ever. So when shopping for products to blow off, clean, move, and cool, look not only at the performance data, design and workmanship – all which are important of course – but also refer to the quality and type of material used in construction. You can also refer to this article on how to avoid galvanic corrosion. Remember that materials used and how they are put together does make a difference.